WATER ETECH SOLUTIONS
Industrial Use
The industrial use of WTS consists of multiple applications:
1. Cooling Tower
Water ETech Solutions (WTS) on the Cooling
Tower Filler
- No chemical feed to be required
- Much less blow down to be required
- No softener or RO to be required for makeup water
- Removal of the existing scales and no further formation of scales
- Increased efficiency of heat exchange
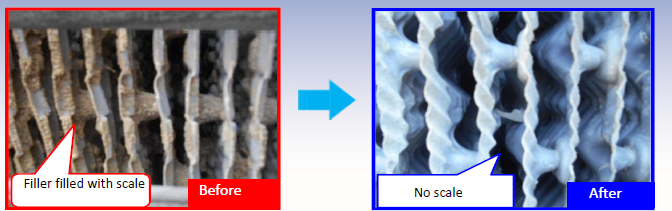
Scale Removal by using Water eTech Solutions (WTS)
Red Rust exists as Fe2O3(Ⅲ) in water
_ The treated water with our device reacts and is reduced into Fe(OH)2 as follows:
H+ + O2 + e- → HO2
Fe(OH)3 + HO2 → Fe(OH)2+H2O+O2
_ Red rust is dissolved by activated water and reduced Fe(OH)2 is combined with
Fe(OH)3,and it makes black rust asFe(OH)8 in water.
_ Fe(OH)2 + 2Fe(OH)3 → Fe(OH)8 (Black rust in water)
_ → 4 H2O +FeO4 (Black rust in the air)
Scale issues have been one of the most serious water related issues. The causes for the formation of scales are ionized minerals like Si, Ca and Mg ( the ultimate cause for the
formation of scales, particularly “ionized Silica can be a big cause”), oxidized iron & zinc and other impurities. Scale can be formed by the reaction of ionized minerals like Si, Ca and Mg with oxidized material including iron, zinc and other impurities. It is more possible for scales to be formed at a higher temperature and high PH.

2. Boiler
Water eTech Solutions (WTS) use in the Boiler
- Removal of the existing scales and no further formation of scales
- Increased heat efficiency
- Increased efficiency of fuel consumption
- No softener to be required

